- 22 -
Appendices
6. Appendices
6.1. Appendix 1- Volumetric versus Mass Flow
Mass flow measures just what it says, the mass or weight of the gas flowing through the
instrument. Mass flow (or weight per unit time) units are given in pounds per hour (lb/hour),
kilograms per sec (kg/sec) etc. When your specifications state units of flow to be in mass
units, there is no reason to reference a temperature or pressure. Mass does not change based
on temperature or pressure.
However, if you need to see your results of gas flow in volumetric units, like liters per minute,
cubic feet per hour, etc. you must consider the fact that volume DOES change with
temperature and pressure.
A mass flow meter measures MASS (grams) and then converts mass to volume. To do this the
density (grams/liter) of the gas must be known and this value changes with temperature and
pressure.
When you heat a gas, the molecules have more energy and they move around faster, so when
they bounce off each other, they become more spread out, therefore the volume is different
for the same number of molecules.
Think about this:
The density of Air at 0° C is 1.29 g/liter
The density of Air at 25C is 1.19 g/liter
The difference is 0.1 g/liter. If you are measuring flows of 100 liters per minute, and you don’t
use the correct density factor then you will have an error of 10 g/minute!
Volume also changes with pressure. Think about a helium balloon with a volume of 1 liter. If
you could scuba dive with this balloon and the pressure on it increases. What do you think
happens to the weight of the helium? It stays the same. What would happen to the volume (1
liter)? It would shrink.
Why is the word standard included with the volume terms liters and cubic feet in mass flow
applications?
A mass flow meter measures mass …and we know we can convert to volume.
To use density we must pick one (or standard) temperature and pressure to use in our
calculation. When this calculation is done,
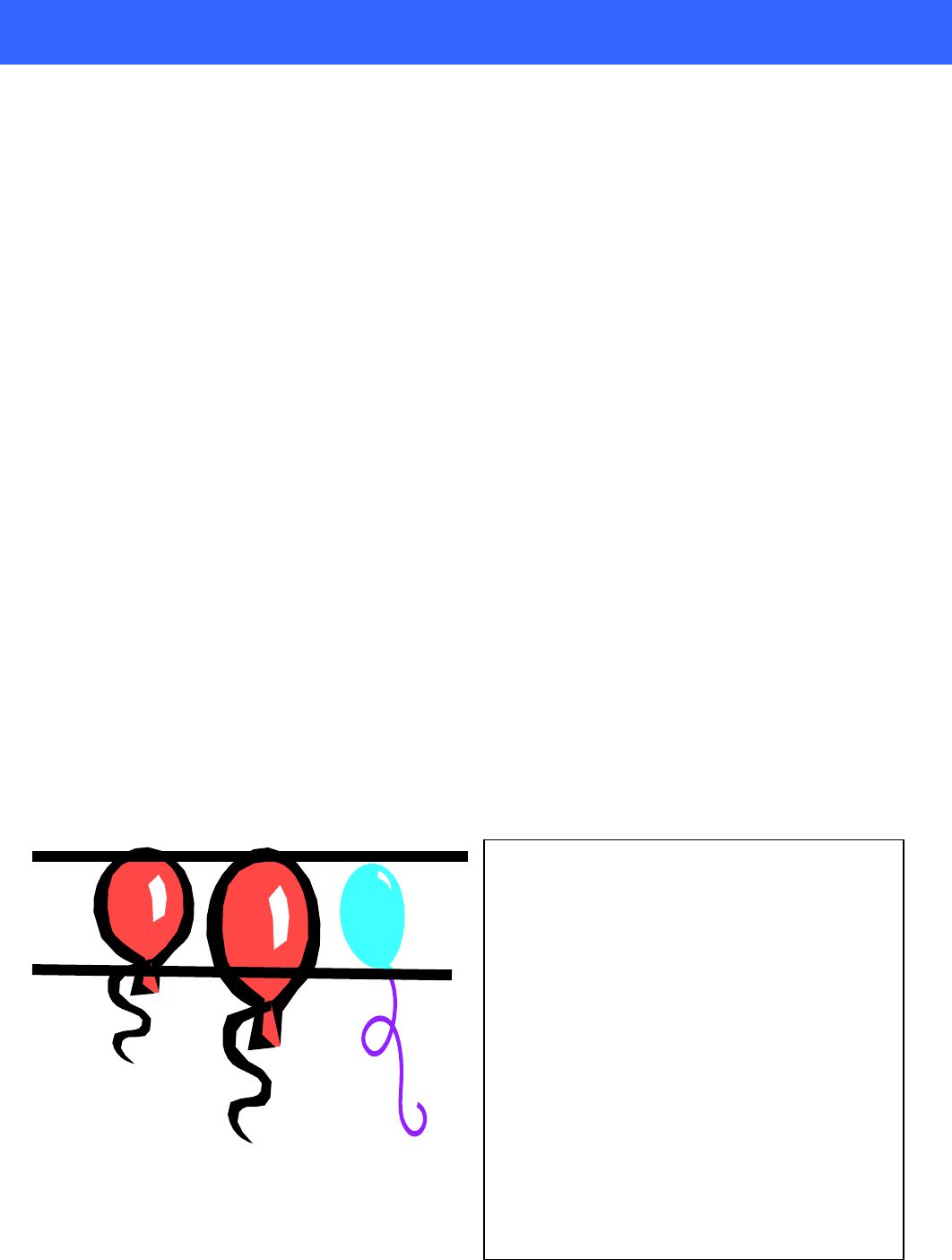
Using the example to the left, we can see a
standard liter can be defined differently. The
first balloon contains 0.179 grams of Helium at
0 ° C and 760 Torr (density of 0.179
grams/liter). Heat up that balloon to room
temperature and the volume increases, but the
mass has not changed – but the volume is not 1
liter anymore, it is 1.08 liters.
So to define a standard liter of Helium at 25 C,
we must extract only one liter from the second
balloon and that liter weighs only 0.175 grams.
If a mass flow meter is set up for STP at 0 C and
760 Torr, when it measures 0.179 grams of He,
it will give you results of 1 SLM.
If a second meter is set up for STP at 25 C and
760 Torr, when it measures 0.164 grams, it will
give results of 1 SLM.
0° C
0..179 grams/1
liter
1 Liter
1.08 Liter 1 Liter
25° C
0.164 grams
25 C
0.179 g/1.08
liters