2. Professional / Lay User Evaluation
A Patient Use evaluation was conducted at two diabetes
clinics. Lay users with diabetes were given product
instructions with no training. After reviewing the
instructions, the users performed fingersticks and glucose
assays using the Ascensia™ C
ONTOUR™ System. Two reagent
lots were evaluated at each clinic; one was common to both
sites. After the user’s self test, the attending HCP performed
Ascensia C
ONTOUR System assays from the lay user’s
25
95% Confidence Interval
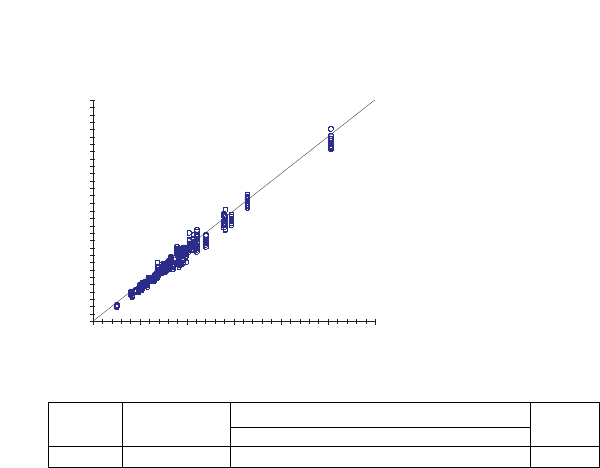
ny = Slope / Intercept r
746 1.01x – 0.49 1.00 to 1.03 –0.58 to –0.39 0.992
0
5
10
15
20
25
30
051015 20 25 30
YSI Plasma Glucose Result (mmol/L)
Ascensia C
ONTOUR
Result (mmol/L)
y=x
Passing and Bablok Regression Statistics / Pearson correlation


















