Stratos LV/LV-T Technical Manual 27
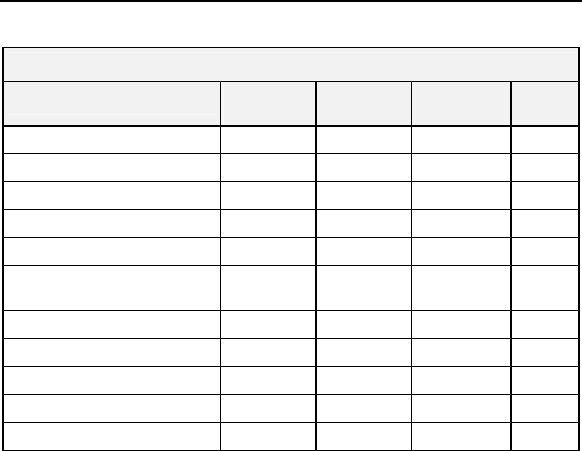
Table 3: Current Cardiac Medications at Enrollment
Drug Category
Group 1
N=42
Group 2
N=50
Group 3
N=25
P-
value*
Anti-Arrhythmics 12 (28.6%) 10 (20.4%) 4 (16.0%)
0.480
Rate Control Medications 32 (76.2%) 43 (87.8%) 20(80.0%)
0.462
Anti-thrombic Agents 17 (40.5%) 19(38.8%) 11 (44.0%)
0.863
Anti-Coagulants 36 (85.7%) 40 (81.6%) 22 (88.0%)
0.686
ACE Inhibitors 16 (38.1%) 16 (32.7%) 8 (32.0%)
0.848
Angiotensin-Receptor
Blockers
10 (23.8%) 7 (14.3%) 4 (16.0%) 0.491
Diuretics 30 (71.4%) 34 (69.4%) 13 (52.0%)
0.255
Inotropes 1 (2.4%) 2 (4.1%) 0 (0.0%)
0.803
Nitrates 3 (7.1%) 6 (12.2%) 2 (8.0%)
0.714
Beta-Blockers for CHF 6 (14.3%) 9 (18.4%) 4 (16.0%)
0.947
Other 23 (54.8%) 26 (53.1%) 14 (56.0%)
0.941
*Chi-Square test (2-sided)
Safety and Effectiveness Results
A total of 118 patients were enrolled in the AVAIL CLS/CRT
clinical study at 20 sites:
There were 43 Group 1, 50 Group 2, and 25 Group 3 patients in
this prospective, multi-center, randomized clinical study. For
Group 1, there were 33 successful implants (76.7%) of the
Protos DR/CLS system. For Groups 2 and 3, there were 44 and
21 successful implants (88.0% and 84.0%) respectively of the
Stratos LV CRT-P system.


















