Page 5 of 24
English
Leica Biosystems Leica HER2 FISH System - 30 Test Instructions for Use TA9217 EN-CE-Rev_D 08/04/2013
English
The Leica HER2 FISH System - 30 Test is for use only on the Leica BOND-MAX and BOND-III
System.
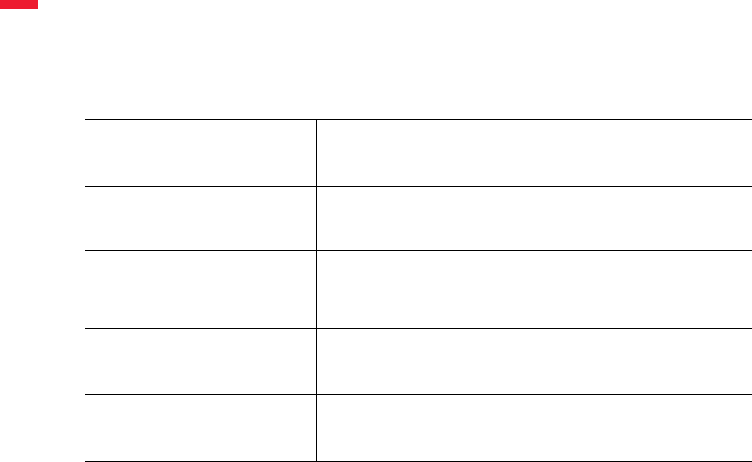
Components Provided
The materials listed below (Table 1) are sufcient to stain 30 tests (30 slides stained with LSI
HER2/CEP17 Dual Probe).
LSI HER2/CEP17 Probe
6.6 mL
Contains ready-to-use LSI HER2/CEP17 Dual Probe.
Contains <60% (v/v) formamide.
Post Hybridization Wash 2
9 mL
Contains ready-to-use post hybridization wash solution.
Contains <50% (v/v) formamide.
Leica BOND Enzyme
Concentrate 2
1 mL
Contains Proteinase K solution at 1.7 mg/mL.
Leica BOND Enzyme Diluent
65 mL
Contains Enzyme Diluent.
Leica BOND Open Container
3 x 7 mL
BOND Open Container used for Enzyme 5.
Table 1: Leica HER2 FISH System - 30 Test Components
Refer to individual MSDS for further product safety information, available from
www.LeicaBiosystems.com/TA9217-IFU
Directions For Use
All reagents supplied are formulated specically for use with this assay and lot numbers are
specic for each Leica HER2 FISH System - 30 Test. For the assay to be valid, no substitutions
should be made.
Storage and Stability
Store at 2–8 °C. Do not freeze. Return to 2–8 °C immediately after use. Any deviation from these
conditions will invalidate the assay. Ensure the Leica HER2 FISH System - 30 Test is used within
its designated expiry date. The signs indicating contamination and/or instability of the Leica
HER2 FISH System - 30 Test are turbidity of the solutions (except for the probe solution) and
odor development. The user must verify storage conditions other than those specied above.
Specimen Preparation
Standard methods of tissue processing should be used for all specimens (19). It is recommended
that tissues are prepared in formalin-based xatives and are routinely processed and parafn-
embedded. For example, specimens should be sampled at a thickness of 3–4 mm and xed
for 18–24 hours in 10% neutral-buffered formalin. The tissues should then be dehydrated in a
series of alcohols and cleared through xylene, followed by impregnation with molten parafn
wax, held at no more than 60 °C. Tissue specimens should be sectioned between 4–6 µm.


















