English
Page 16 of 24
English
Leica Biosystems Leica HER2 FISH System - 30 Test Instructions for Use TA9217 EN-CE-Rev_D 08/04/2013
2x2 Concordance Results Leica BOND-III System
Data was grouped as negative (<2.00) or positive (≥2.00) for a 2x2 analysis The observed
agreement for 300 samples between the two tests in a 2x2 analysis show a concordance of
99.67% (299/300) with a 95% CI of 98.16–99.99% for the Leica BOND-III System.
The percentage Positive Agreement (sensitivity) or the ability of the Leica HER2 FISH System
- 30 Test to correctly identify Abbott Molecular PathVysion HER-2 DNA Probe Kit assay positive
cases (the percentage of specimens scored positive by both the Leica HER2 FISH System -
30 Test and manual Abbott Molecular PathVysion HER-2 DNA Probe Kit out of all the Abbott
Molecular PathVysion HER-2 DNA Probe Kit positive cases) was 99.03% (102/103).
The percentage Negative Agreement (specicity) or the ability of the test to correctly identify
Abbott Molecular PathVysion HER-2 DNA Probe Kit negative cases (the percentage of
specimens scored negative by the Leica HER2 FISH System - 30 Test and Abbott Molecular
PathVysion HER-2 DNA Probe Kit out of all the Abbott Molecular PathVysion HER-2 DNA Probe
Kit negative cases) was 100% (197/197). See Table 5.
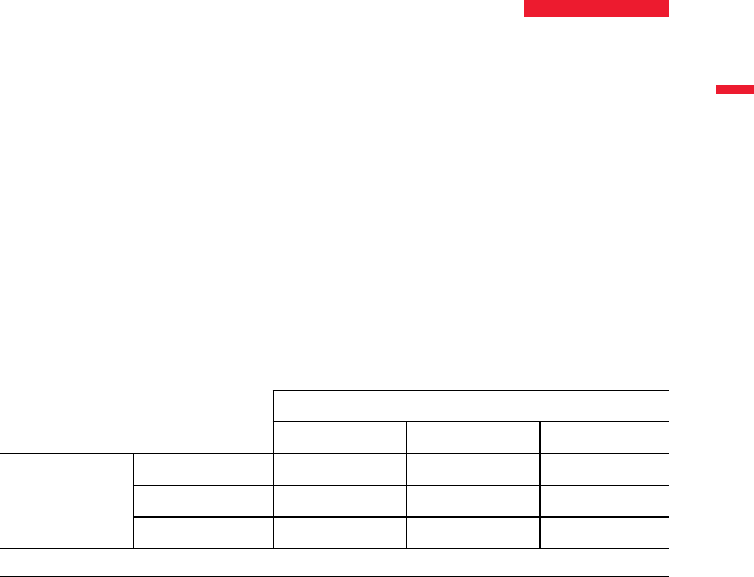
Abbott Molecular PathVysion HER-2 DNA Probe Kit
Negative (<2.0) Positive (≥2.0) Totals
Leica HER2 FISH
System - 30 Test
Leica BOND-III
Negative (<2.0)
197 1 198
Positive (≥2.0)
0 102 102
Totals
197 103 300
Overall Concordance (95% CI) = 99.67% (98.16 – 99.99%).
Table 5. 2x2 concordance of Leica HER2 FISH System - 30 Test on the Leica BOND-III System with Abbott Molecular
PathVysion HER-2 DNA Probe Kit.
In conclusion, the data generated in this study demonstrates that the Leica HER2 FISH System
- 30 Test can be used as an aid in the assessment of patients for whom Herceptin (trastuzumab)
treatment is being considered, based upon its high concordance with Abbott Molecular
PathVysion HER-2 DNA Probe Kit, a previously approved diagnostic test for this indication.
