Appendix: How Infrared Thermometry Works
A
A-2
Blackbody
When thermal radiation falls on an object, part of the energy is
transmitted through the object, part is reflected and part is
absorbed. A blackbody is defined as an ideal object that absorbs all
the radiation incident upon it. The best example of a real object that
acts like a blackbody is a small hole drilled deep into a large opaque
cavity. Thermal radiation entering the cavity is internally reflected
and has little chance of escaping the cavity before it is fully
absorbed.
Emissivity is defined as the ratio of energy radiated by an object to
that of the energy radiated by a blackbody. By definition, the
emissivity of a blackbody is 1. Most objects are considered gray
objects with an emissivity between 0 and 1. Various emissivities for
common materials are shown in Appendix B.
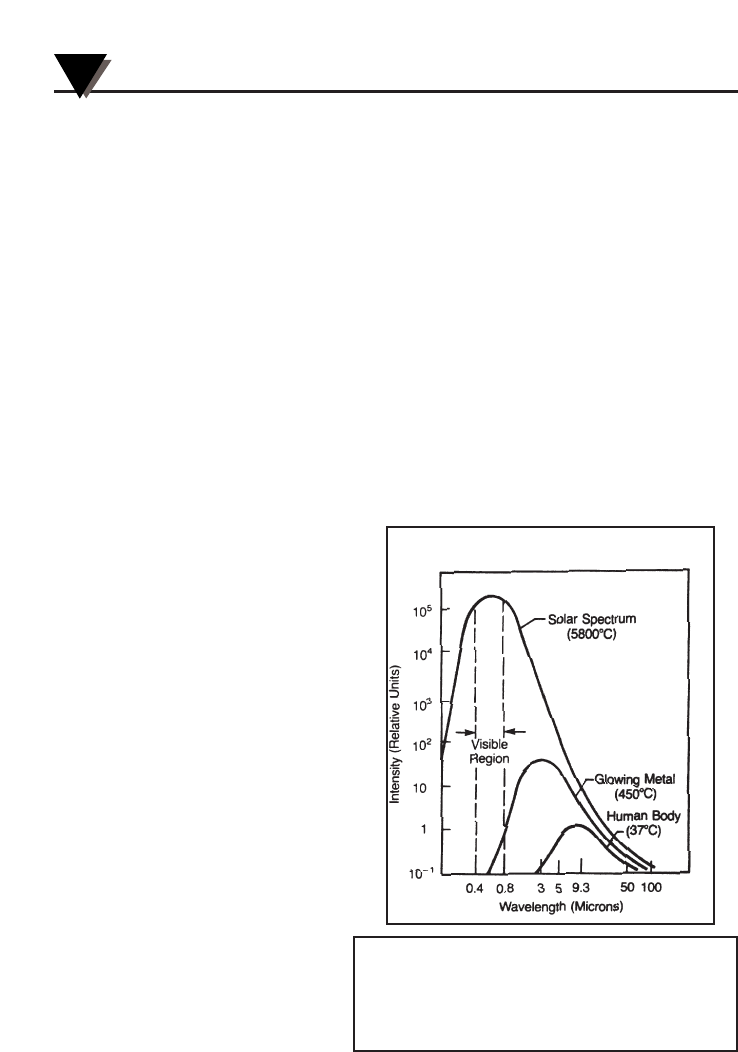
Spectral Distribution
Objects radiate energy at
different wavelengths, but
not with constant intensity
at each wavelength. Figure
A-2 shows the energy
radiated by a blackbody at
various temperatures as a
function of wavelength. As
a body is heated, the
intensity of the radiated
energy increases and the
peak of the curve shifts
towards the shorter
wavelength end of the
spectrum. The total area
under a spectral
distribution curve is
proportional to the total
energy radiated by the
blackbody at a given
temperature.
Figure A-2. Blackbody Spectral Distribution
Relative emission from a blackbody versus wavelength.
The area under the curve corresponds to the total ener-
gy, and is proportional to the absolute temperature to
the 4th power. The peak of the spectral distribution
curve shifts to shorter wavelengths as the temperature
increases.


















