67
For best accuracy, it is recommended to calibrate the instrument frequently.
In addition a pH calibration should be performed:
a) Whenever the pH electrode or the batteries are replaced
b) At least once a month
c) After testing aggressive chemicals
d) When extreme accuracy is required
PREPARATION
Pour small quantities of pH 7.01 and pH 4.01 (or pH 10.01) solution into
two clean beakers. For an accurate calibration, use two beakers for each
buffer solution, the first one for rinsing the electrode and the second one for
calibration.
For accurate readings, use pH 7.01 & pH 4.01 buffers if you are going to
measure acidic solutions, or pH 7.01 & pH 10.01 for alkaline samples.
PROCEDURE
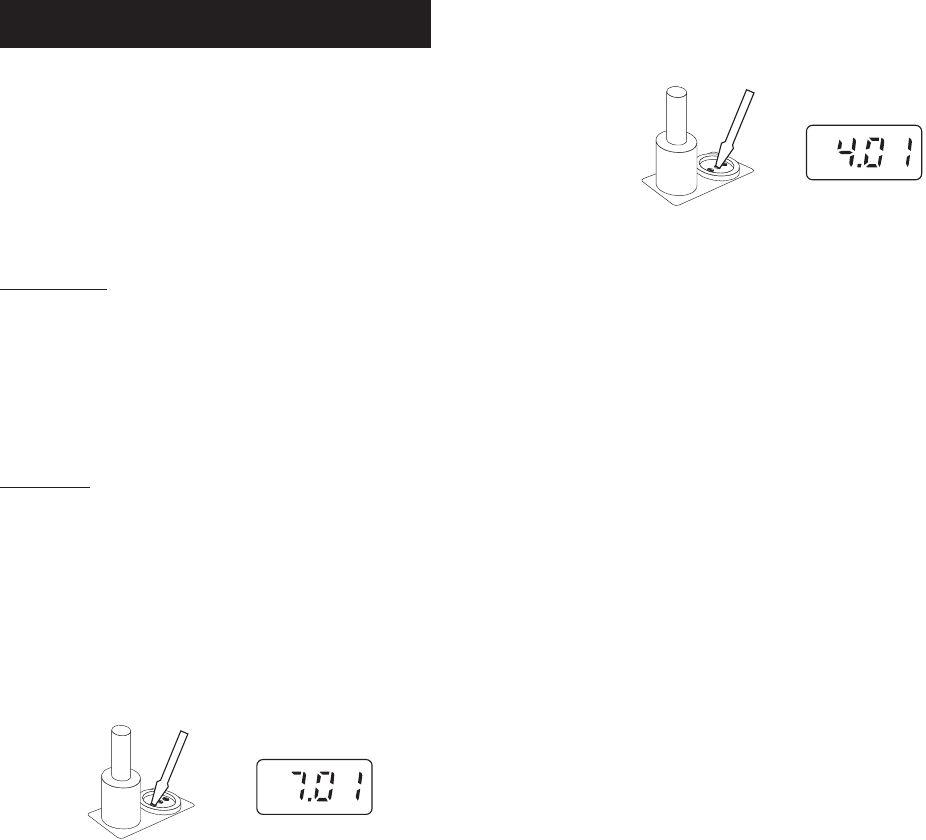
• Remove the protective cap from the electrode, rinse it with some pH
7.01 solution, then dip it (at least 4 cm/1½") in pH 7.01 buffer. Stir
briefly and wait for the reading to stabilize.
• To access the calibration trimmers, unscrew the protective cap on the top
of the meter, near the electrode connector.
• Adjust the OFFSET trimmer until the LCD shows the buffer pH value at
the current temperature (see “pH values at various temperatures”
table). Press RANGE to read the buffer temperature.
• Rinse the electrode and dip it into pH 4.01 (or pH 10.01) buffer. Stir
gently and wait for the display to stabilize.
• Adjust the SLOPE trimmer until the LCD displays the buffer pH value at
the measured temperature.
CALIBRATION
Note: The meter also allows the user to calibrate the offset point of the
temperature sensor. For this purpose, immerse the HI 1217D
electrode in a solution together with a high precision reference
thermometer. Then turn the temperature calibration trimmer (#5
at page 4) until the meter displays the temperature of the solution.
pH
• pH calibration is now complete. Replace the trimmer cover and tighten
it to ensure a proper seal against water and humidity ingress.
pH









